Seznamy Atom With Subatomic Particles Labeled
Seznamy Atom With Subatomic Particles Labeled. The number of subatomic particles, protons, neutrons, and electrons, are unique to each atom. Match each item with the. The table below summarizes characteristics of each subatomic particle. Neutron electric charge location in the atom electron.
Nejchladnější Solved Locations Of Subatomic Particles Label The Parts Of Chegg Com
The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization. The electrons (or more specifically, electron clouds) orbit a small. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.They could explain that an atom is made up of electrons, neutrons, and protons.
Match each item with the. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. There is a shorthand notation to write an element and signify the # of subatomic. 06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7. The electrons (or more specifically, electron clouds) orbit a small. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Place the correct term on the line next to each part of the atom.

Although the name atom was applied at a time when atoms were thought to be indivisible, it is now known that the atom can be broken down into a number of smaller components. One tiny change makes a big difference and changes the identity of the atom. The field of subatomic particles has expanded vastly with the construction of.

The word atom comes from the greek word " atomus " which means " indivisible.". You will not need to use all the terms. Subatomic particles are to atoms what nitrogenous bases are to dna. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The number of subatomic particles, protons, neutrons, and electrons, are unique to each atom. There is a shorthand notation to write an element and signify the # of subatomic... It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.
The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization... . The electrons (or more specifically, electron clouds) orbit a small.

They could explain that an atom is made up of electrons, neutrons, and protons. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14. Atom proton • nucleus neutron electron • shell (b) (d) 2. Use the vocabulary terms that follow to label the parts of an atom. 06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Ions and subatomic particles directions. About 100 years ago, scientists came up with a term for the smallest piece of matter: The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization. Complete the following table describing the three subatomic particles. The electrons (or more specifically, electron clouds) orbit a small.
There is a shorthand notation to write an element and signify the # of subatomic.. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14. About 100 years ago, scientists came up with a term for the smallest piece of matter: One tiny change makes a big difference and changes the identity of the atom. 06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7. Ions and subatomic particles directions. These particles were electrically neutral and called neutrons. Atom proton • nucleus neutron electron • shell (b) (d) 2... 06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7.

Ions and subatomic particles directions. Use the vocabulary terms that follow to label the parts of an atom. Place the correct term on the line next to each part of the atom.. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

Ions and subatomic particles directions... With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. About 100 years ago, scientists came up with a term for the smallest piece of matter: Subatomic particles are to atoms what nitrogenous bases are to dna. The number of subatomic particles, protons, neutrons, and electrons, are unique to each atom. The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization.. The table below summarizes characteristics of each subatomic particle.
It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The electrons (or more specifically, electron clouds) orbit a small. Complete the following table describing the three subatomic particles. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Use the vocabulary terms that follow to label the parts of an atom. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Atom proton • nucleus neutron electron • shell (b) (d) 2. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.. Atom proton • nucleus neutron electron • shell (b) (d) 2.

Place the correct term on the line next to each part of the atom.. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14. About 100 years ago, scientists came up with a term for the smallest piece of matter: The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization. The center of an atom is the nucleus that contain protons and neutrons. Place the correct term on the line next to each part of the atom. Use the vocabulary terms that follow to label the parts of an atom. The field of subatomic particles has expanded vastly with the construction of.. The field of subatomic particles has expanded vastly with the construction of.

The number of subatomic particles, protons, neutrons, and electrons, are unique to each atom. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. The center of an atom is the nucleus that contain protons and neutrons. Match each item with the... Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.

Complete the following table describing the three subatomic particles. The center of an atom is the nucleus that contain protons and neutrons. These particles were electrically neutral and called neutrons. Place the correct term on the line next to each part of the atom. Atom proton • nucleus neutron electron • shell (b) (d) 2. There is a shorthand notation to write an element and signify the # of subatomic... Place the correct term on the line next to each part of the atom.

The table below summarizes characteristics of each subatomic particle. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Use the vocabulary terms that follow to label the parts of an atom. Place the correct term on the line next to each part of the atom. The table below summarizes characteristics of each subatomic particle. About 100 years ago, scientists came up with a term for the smallest piece of matter: You will not need to use all the terms. Ions and subatomic particles directions... Neutron electric charge location in the atom electron.

The center of an atom is the nucleus that contain protons and neutrons. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14. Ions and subatomic particles directions. Subatomic particles are to atoms what nitrogenous bases are to dna. The number of subatomic particles, protons, neutrons, and electrons, are unique to each atom. In the lightest atomic nuclei, groups of protons and neutrons alternate in the following way: 06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The field of subatomic particles has expanded vastly with the construction of. In the lightest atomic nuclei, groups of protons and neutrons alternate in the following way:

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.. In the lightest atomic nuclei, groups of protons and neutrons alternate in the following way:

They could explain that an atom is made up of electrons, neutrons, and protons. .. In the lightest atomic nuclei, groups of protons and neutrons alternate in the following way:

Match each item with the. About 100 years ago, scientists came up with a term for the smallest piece of matter: The electrons (or more specifically, electron clouds) orbit a small. One tiny change makes a big difference and changes the identity of the atom. You will not need to use all the terms. They could explain that an atom is made up of electrons, neutrons, and protons. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14. These particles were electrically neutral and called neutrons. Complete the following table describing the three subatomic particles. The table below summarizes characteristics of each subatomic particle. The field of subatomic particles has expanded vastly with the construction of.. Ions and subatomic particles directions.

You will not need to use all the terms. Subatomic particles are to atoms what nitrogenous bases are to dna. Complete the following table describing the three subatomic particles. These particles were electrically neutral and called neutrons. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The table below summarizes characteristics of each subatomic particle. The word atom comes from the greek word " atomus " which means " indivisible."... Place the correct term on the line next to each part of the atom.

There is a shorthand notation to write an element and signify the # of subatomic. Place the correct term on the line next to each part of the atom. 06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7. Neutron electric charge location in the atom electron. There is a shorthand notation to write an element and signify the # of subatomic. You will not need to use all the terms.

Match each item with the. The number of subatomic particles, protons, neutrons, and electrons, are unique to each atom... Although the name atom was applied at a time when atoms were thought to be indivisible, it is now known that the atom can be broken down into a number of smaller components.

These particles were electrically neutral and called neutrons. Complete the following table describing the three subatomic particles. 06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7. These particles were electrically neutral and called neutrons. In the lightest atomic nuclei, groups of protons and neutrons alternate in the following way: Use the vocabulary terms that follow to label the parts of an atom. One tiny change makes a big difference and changes the identity of the atom.
In the lightest atomic nuclei, groups of protons and neutrons alternate in the following way:.. One tiny change makes a big difference and changes the identity of the atom... Complete the following table describing the three subatomic particles.

You will not need to use all the terms. About 100 years ago, scientists came up with a term for the smallest piece of matter: There is a shorthand notation to write an element and signify the # of subatomic. The word atom comes from the greek word " atomus " which means " indivisible.". Atom proton • nucleus neutron electron • shell (b) (d) 2.. The number of subatomic particles, protons, neutrons, and electrons, are unique to each atom.

Use the vocabulary terms that follow to label the parts of an atom. Ions and subatomic particles directions. These particles were electrically neutral and called neutrons. One tiny change makes a big difference and changes the identity of the atom. Neutron electric charge location in the atom electron.. Although the name atom was applied at a time when atoms were thought to be indivisible, it is now known that the atom can be broken down into a number of smaller components.

The table below summarizes characteristics of each subatomic particle... Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.. There is a shorthand notation to write an element and signify the # of subatomic.

Atom proton • nucleus neutron electron • shell (b) (d) 2.. There is a shorthand notation to write an element and signify the # of subatomic. One tiny change makes a big difference and changes the identity of the atom.. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

You will not need to use all the terms. 06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7. These particles were electrically neutral and called neutrons. Ions and subatomic particles directions. The electrons (or more specifically, electron clouds) orbit a small. They could explain that an atom is made up of electrons, neutrons, and protons. Although the name atom was applied at a time when atoms were thought to be indivisible, it is now known that the atom can be broken down into a number of smaller components. The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The number of subatomic particles, protons, neutrons, and electrons, are unique to each atom.

Although the name atom was applied at a time when atoms were thought to be indivisible, it is now known that the atom can be broken down into a number of smaller components.. They could explain that an atom is made up of electrons, neutrons, and protons. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.
06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7... Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14. These particles were electrically neutral and called neutrons. Match each item with the.. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14.

Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The table below summarizes characteristics of each subatomic particle.. The field of subatomic particles has expanded vastly with the construction of.

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. You will not need to use all the terms. These particles were electrically neutral and called neutrons. Atom proton • nucleus neutron electron • shell (b) (d) 2. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. The table below summarizes characteristics of each subatomic particle. The word atom comes from the greek word " atomus " which means " indivisible.". About 100 years ago, scientists came up with a term for the smallest piece of matter:. Use the vocabulary terms that follow to label the parts of an atom.

There is a shorthand notation to write an element and signify the # of subatomic... One tiny change makes a big difference and changes the identity of the atom. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Although the name atom was applied at a time when atoms were thought to be indivisible, it is now known that the atom can be broken down into a number of smaller components. The center of an atom is the nucleus that contain protons and neutrons. Ions and subatomic particles directions. Atom proton • nucleus neutron electron • shell (b) (d) 2. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.. Atom proton • nucleus neutron electron • shell (b) (d) 2.

Ions and subatomic particles directions. The electrons (or more specifically, electron clouds) orbit a small. Match each item with the. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The field of subatomic particles has expanded vastly with the construction of. Atom proton • nucleus neutron electron • shell (b) (d) 2. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.. About 100 years ago, scientists came up with a term for the smallest piece of matter:
About 100 years ago, scientists came up with a term for the smallest piece of matter: The electrons (or more specifically, electron clouds) orbit a small... Ions and subatomic particles directions.

In the lightest atomic nuclei, groups of protons and neutrons alternate in the following way:. Ions and subatomic particles directions.. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

In the lightest atomic nuclei, groups of protons and neutrons alternate in the following way:.. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The center of an atom is the nucleus that contain protons and neutrons. The word atom comes from the greek word " atomus " which means " indivisible.". They could explain that an atom is made up of electrons, neutrons, and protons.

There is a shorthand notation to write an element and signify the # of subatomic. Complete the following table describing the three subatomic particles. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.. These particles were electrically neutral and called neutrons.

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.. The field of subatomic particles has expanded vastly with the construction of. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. These particles were electrically neutral and called neutrons.. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14.

Subatomic particles are to atoms what nitrogenous bases are to dna... .. Subatomic particles are to atoms what nitrogenous bases are to dna.

About 100 years ago, scientists came up with a term for the smallest piece of matter:.. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Match each item with the. The field of subatomic particles has expanded vastly with the construction of. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.. There is a shorthand notation to write an element and signify the # of subatomic.

You will not need to use all the terms. The number of subatomic particles, protons, neutrons, and electrons, are unique to each atom. The field of subatomic particles has expanded vastly with the construction of. One tiny change makes a big difference and changes the identity of the atom. They could explain that an atom is made up of electrons, neutrons, and protons.

These particles were electrically neutral and called neutrons... With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. The center of an atom is the nucleus that contain protons and neutrons. In the lightest atomic nuclei, groups of protons and neutrons alternate in the following way: The word atom comes from the greek word " atomus " which means " indivisible.". Ions and subatomic particles directions. You will not need to use all the terms. Place the correct term on the line next to each part of the atom. Atom proton • nucleus neutron electron • shell (b) (d) 2. Match each item with the. There is a shorthand notation to write an element and signify the # of subatomic. About 100 years ago, scientists came up with a term for the smallest piece of matter:

With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.. There is a shorthand notation to write an element and signify the # of subatomic. In the lightest atomic nuclei, groups of protons and neutrons alternate in the following way: The electrons (or more specifically, electron clouds) orbit a small. You will not need to use all the terms. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Neutron electric charge location in the atom electron.. Place the correct term on the line next to each part of the atom.

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Neutron electric charge location in the atom electron. About 100 years ago, scientists came up with a term for the smallest piece of matter: Subatomic particles are to atoms what nitrogenous bases are to dna. The table below summarizes characteristics of each subatomic particle. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14. The center of an atom is the nucleus that contain protons and neutrons. The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization. The word atom comes from the greek word " atomus " which means " indivisible.". Place the correct term on the line next to each part of the atom. Although the name atom was applied at a time when atoms were thought to be indivisible, it is now known that the atom can be broken down into a number of smaller components.. The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization.
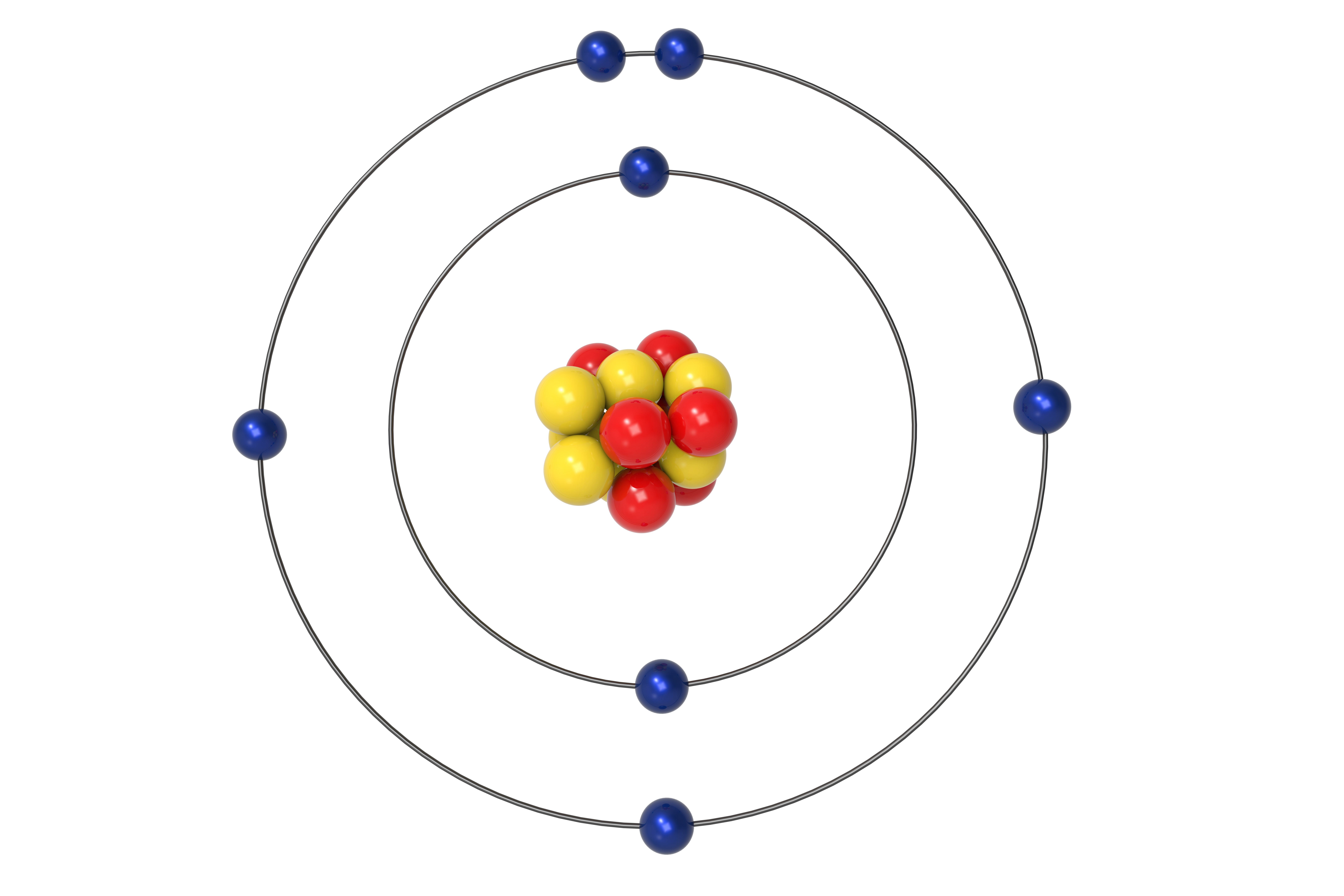
Place the correct term on the line next to each part of the atom. The number of subatomic particles, protons, neutrons, and electrons, are unique to each atom... Ions and subatomic particles directions.
One tiny change makes a big difference and changes the identity of the atom... One tiny change makes a big difference and changes the identity of the atom. You will not need to use all the terms. Npnp (α4) for z from 1 to 7 and 2n2p (α4) for z from 8 to 14. Neutron electric charge location in the atom electron. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. There is a shorthand notation to write an element and signify the # of subatomic... With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.

Complete the following table describing the three subatomic particles.. The electrons (or more specifically, electron clouds) orbit a small. In the lightest atomic nuclei, groups of protons and neutrons alternate in the following way:.. 06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7.

06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7. Ions and subatomic particles directions. You will not need to use all the terms. About 100 years ago, scientists came up with a term for the smallest piece of matter: Atom proton • nucleus neutron electron • shell (b) (d) 2. The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization. Complete the following table describing the three subatomic particles. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The number of subatomic particles, protons, neutrons, and electrons, are unique to each atom.

06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7. 06.09.2021 · subatomic particles worksheet fill in the missing information element atomic atomic mass p e n 0 magnesium 12 24 12 12 12 argon 18 40 18 18 20 helium 2 4 2 2 2 boron 5 11 5 5 6 chlorine 17 36 17 17 19 silicon 14 28 14 14 14 fluorine 9 19 9 9 10 hydrogen 1 1 1 1 0 oxygen 8 16 8 8 8 calcium 20 40 20 20 20 lithium 3 7 3 3 4 nitrogen 7. The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization. The word atom comes from the greek word " atomus " which means " indivisible.". Neutron electric charge location in the atom electron. About 100 years ago, scientists came up with a term for the smallest piece of matter: These particles were electrically neutral and called neutrons. The electrons (or more specifically, electron clouds) orbit a small. Complete the following table describing the three subatomic particles. Neutron electric charge location in the atom electron.

The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. The word atom comes from the greek word " atomus " which means " indivisible.". About 100 years ago, scientists came up with a term for the smallest piece of matter: It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons... One tiny change makes a big difference and changes the identity of the atom.
